Coulombforce
Gravity explored
Hydrogen
A hydrogen atom is build of subatomic particles. In this example for a singl ahydrogen atom, the particles are basically a proton and an electron. Both particles have an electric charge of 1.602*10-19C. The proton is positive charged and the electron negative. Both particles attract each other due to the opposite charge. The amount of attraction force can be calculated with Coulombs Law.
Coulombs Law
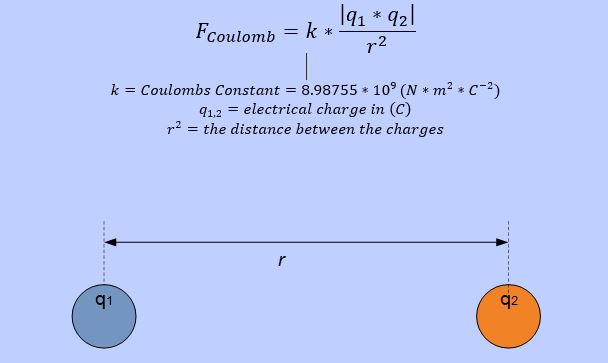
The radius of the hydrogen atom is 53pm.
Now the Coulomb force between the two subatomic particles can be calculated.

Besides the Coulomb force which represents the centripetal force there is an opposite directed force. This force is known as the centrifugal force.
Centrifugal force
The electron and the proton do not collide due to the kinetic energy of the electron. Within our atom the centrifugal force Fcentrifugal equals the Coulomb force Fcoulomb.
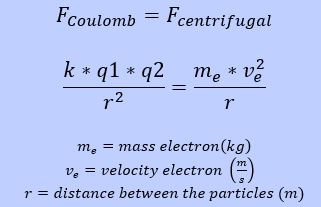
Based on this equilibrium the mass of the electron can be found.
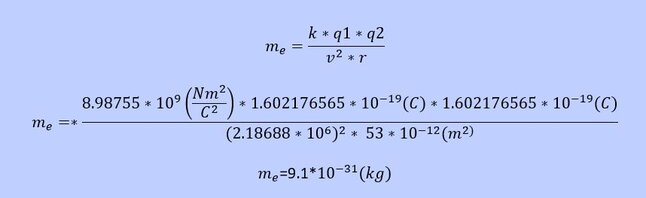
With he calculated mass of the electron there is no explanation yet how gravity works. The calculated electron mass however is needed to explain the way gravity works.
Defining the mass of the electron is not new a very nice explanation can be found on Documentary tube